Principles of nuclear magnetic resonance
1. Nuclear spin

Magnetic resonance diagram
Nuclear magnetic resonance is mainly caused by the spin motion of atomic nuclei. For different nucleuses, the spin motion is different. They can be represented by the nuclear spin quantum number I. There is a certain relationship between the spin quantum number and the mass number and atomic number of atoms, which can be roughly divided into three cases: I with zero nuclei can be seen as a non-spin sphere; I is 1/2 nuclei It can be seen as a spin sphere with uniform charge distribution. I of 1H, 13C, 15N, 19F, and 31P are both 1/2. Their nuclei are all spin spheres with uniform charge distribution; I is greater than 1/2. The nucleus can be seen as a spin ellipsoid with a non-uniform charge distribution.
2. NMR phenomenon
A nucleus is a positively charged particle. A nucleus that cannot spin has no magnetic moment, a nucleus that spins has a circulating current, and a magnetic field is generated to form a magnetic moment (μ). More than half of the nuclei have spins that produce a small magnetic field when they rotate. When an external magnetic field is applied, the energy levels of these atomic nuclei will split, that is, the Zeeman effect.
The Zeeman splitting plot in the external magnetic field B0:
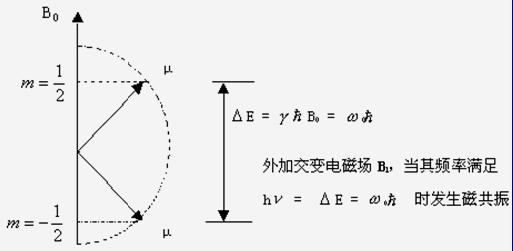 Figure I = 1/2 particle magnetic moment orientation and energy levels in a magnetic field
Figure I = 1/2 particle magnetic moment orientation and energy levels in a magnetic field
The orientation of the microscopic magnetic moment in the external magnetic field is quantized. The nucleus with spin quantum number I can only have 2I+1 orientations under the external magnetic field. Each orientation can be represented by a spin magnetic quantum number m. The relationship between m and I is:
m=I,I-1,I-2...-I, each orientation of the nucleus represents an energy state of the nucleus in the magnetic field.
The nuclear energy arranged in the forward direction is relatively low, and the nuclear energy arranged in the reverse direction is relatively high. The energy difference between them is △E. A core must transition from a low energy state to a high energy state and must absorb the energy of ΔE. The spin nuclei in the external magnetic field are allowed to receive electromagnetic radiation of a certain frequency. When the energy of the radiation is exactly equal to the energy difference between two different orientations of the spin nuclei, the spin nuclei in the low energy state absorb the electromagnetic radiation energy to transition to the high energy state. This phenomenon is called nuclear magnetic resonance, abbreviated as NMR.
At present, the most studied is the 1H nuclear magnetic resonance, and the 13C nuclear magnetic resonance has also seen great development in recent years. The 1H nuclear magnetic resonance is called Proton Magnetic Resonance, abbreviated as PMR, and also expressed as 1H-NMR. Carbon-13 Nuclear Magnetic Resonance (CMR) is also referred to as 13C-NMR.
3.1H NMR Saturation and Relaxation
The spin quantum number of 1H is I=1/2, so the spin magnetic quantum number m=±1/2, that is, the hydrogen nucleus should have two orientations in the external magnetic field. The two orientations of 1H represent two different energy levels, so the 1H nuclear magnetic resonance condition must be such that the electromagnetic radiation frequency is equal to 1H precession frequency.
To make v shot =v0, two methods can be used. One is the fixed magnetic field strength H0, which gradually changes the radiating frequency v-shot of the electromagnetic wave and performs scanning. When v-shot matches H0, nuclear magnetic resonance occurs. Another method is to fix the radiated frequency v of the radiated wave, and then gradually change the magnetic field strength H0 from the low field to the high field. When the H0 matches the v ray, nuclear magnetic resonance also occurs. This method is called sweeping. General instruments use sweeping methods.
Under the effect of external magnetic field, 1H tends to align with the external magnetic field, so the number of nucleus in the low energy state is more than the number of nucleus in the high energy state, but because the energy difference between the two energy levels is small, the former is smaller than the latter. Only a slight advantage. The signal of 1H-NMR is generated by these weakly surplus low energy state nuclear absorption radiant energy of radio frequency electromagnetic waves to jump to high energy levels. If the high-energy nucleus cannot return to the low energy state, then as the transition continues, this weak advantage will be further weakened until it disappears. At this time, the number of 1H nucleus in the low energy state is equal to the number of 1H nucleus in the high energy state. The PMR signal will gradually weaken until it finally disappears. This phenomenon is called saturation.
The 1H core can be converted from a high-energy state to a low-energy state by non-radiative means. This process is called relaxation, and therefore, saturation does not occur under normal test conditions. There are two ways to relax. A nucleus in a high energy state transfers energy to the surrounding molecules through an alternating magnetic field. That is, the system releases energy to the environment and returns itself to a low energy state. This process is called spin lattice relaxation. Its rate is expressed as 1/T2, and T2 is called the spin lattice relaxation time. Spin-lattice relaxation reduces the overall energy of the magnetic core, also known as longitudinal relaxation. Two processes are located within a certain distance. Nuclei with the same precession frequency and different precession orientations interact to exchange energy. The process of changing the precession direction is called spin-spin relaxation. The rate is expressed as 1/T2, and T2 is referred to as the spin-spin relaxation time. Spin-spin relaxation does not reduce the overall energy of the magnetic core, also known as transverse relaxation.
4.13C NMR Abundance and Sensitivity
Natural rich 12C I is zero and there is no NMR signal. The 13C I is 1/2 and has a nuclear magnetic resonance signal. The commonly known carbon spectrum is the 13C NMR spectrum. Since 13C and 1H have the same spin quantum number, the 13C NMR principle is the same as 1H.
The same number of carbon atoms and hydrogen atoms are measured in the same NMR apparatus with the same intensity and temperature as the external magnetic field. The nuclear magnetic resonance signal of carbon is only 1/6000 of hydrogen. This shows that the sensitivity of different atomic nuclei detected in the same magnetic field. differ greatly. The natural abundance of 13C is only 1.108% of 12C. Due to the small sensitivity and low abundance of the test, it is technically more difficult to detect 13C than to detect 1H.
5. Nuclear magnetic resonance apparatus
Currently used NMR instruments have two forms of continuous wave (CN) and pulsed Fourier (PFT) transformation. The CW NMR instrument is mainly composed of magnets, RF transmitters, detectors, amplifiers, and recorders (see Figure 8-5). Magnets are used to generate magnetic fields. There are three main types: permanent magnets, magnetic field strength 14000G, frequency 60MHz; electromagnets, magnetic field strength 23500G, frequency 100MHz; superconducting magnets, frequencies up to 200MHz and up to 500-600MHz. High-frequency instruments have good resolution, high sensitivity, and simple and easy-to-analyze spectra. A scanning coil is provided on the magnet, and it is used to ensure that the magnetic field generated by the magnet is uniform and can continuously and accurately change in a narrow range. RF transmitters are used to generate electromagnetic radiation waves of a fixed frequency. Detectors and amplifiers are used to detect and amplify resonance signals. The recorder plots the resonance signal into a resonance pattern.
In the mid-1970s, a pulsed Fourier NMR instrument appeared. The appearance of the pulsed Fourier NMR instrument enables the 13C nuclear magnetic resonance study to be carried out rapidly.
To realize nuclear magnetic resonance, it is possible to adjust the frequency of the incident electromagnetic wave while keeping the magnetic field constant, or to use a fixed-frequency electromagnetic wave to irradiate the external magnetic field of the sample. The following are the devices that modulate the magnetic field NMR:
(1) Permanent magnets are used to generate a strong external magnetic field. The standard instrument produces a field strength of 1.4 T.
(2) Scanning coils, which are used to make small oscillations in the external magnetic field, so that we can see sharp resonant peaks on the oscilloscope.
(3) Radio frequency oscillators, which are used to generate electromagnetic radiation of a fixed frequency, usually frequency, and the role of this radiating magnetic field.
(4) A detector for detecting the energy absorbed from the oscillator.
A frequency-modulated NMR sample (such as water) is placed in a vial and placed between two poles of the magnet. A coil is wound around the bottle, and a radio frequency oscillator is used to input RF current to it. This current transmits electromagnetic waves of the same frequency to the sample, and its frequency is approximately equal to the frequency corresponding to the external magnetic field. In order to accurately determine the resonance frequency, a frequency-modulated oscillator is used to continuously change the frequency of the RF electromagnetic wave around the resonance frequency. When the frequency of the electromagnetic wave is exactly equal to the resonant frequency, an absorption peak appears at the output of the RF oscillator, which can be seen from the oscilloscope, and the resonant frequency is read out by the frequency counter at the same time.
We have red, blue, green, pink, black, orange, deep yellow, brown colored tape.
Colored packing tape used for sealing, marking, repairing, bulding, different color carton. Colorful tape with smooth surface and high adhesion, it is insolution and waterproof. Our factory could according client's requirements, make custom packing tape.Colored Tape
Colored Tape,Colorful Tape,Colored Packing Tape,Brown Packaging Tape
Dongguan Yalan Packing Materials Co., Ltd. , https://www.yalanpack.com