Analysis and Repair of Common Faults of Potassium Sodium Analyzer
Symptom 1 The noise is loud and the work noise is emitted during work. 
The analysis and overhaul of this kind of phenomenon are generally caused by problems on the circuit. For example, the circuit connection is not firm, the contact is bad, the intermittent power supply is generated, the power supply voltage is higher than the allowable value, and the device has an abnormal working state, which can cause the equipment to work noise. After careful inspection of the measuring circuit, the circuit has been eliminated. Then, after asking the clinical staff in detail, they said that since the recent replacement of the sampling needle, there was a "squeaky" noise failure. Because the circuit problem was previously eliminated, it is natural to transfer the cause of the fault to the replacement needle. In order to determine the cause of the problem as soon as possible, the newly replaced sampling pin was replaced, the old pin was reinstalled, and the power operating device was turned on. The “click†sound of the previous operation disappeared and the machine operation sound was normal. Comparing the old and new sampling needles carefully, it was found that the manufacturing processes of the old and new needles are quite different. The old needle has a high degree of finish and the new needle has a low finish. Using a caliper to measure the diameter, the new needle diameter was 0.1 mm more than the diameter of the old needle from the mouth to the middle. In order to troubleshoot the problem in a timely manner and ensure work, in the absence of new sampling needles in line with the use of specifications, the new sample needle was uniformly ground to 0.08 mm using high-grade sandpaper, and the device was tested, and the equipment failed. No sound again, troubleshooting. 
The direct cause of the failure is analyzed as follows: The diameter of the new sampling needle increases, resulting in smaller pinhole clearance and increased running resistance. When the machine is running, the rotating speed of the motor inside the analyzer is not synchronized with the speed of the up and down movement of the sampling needle, so that a chattering noise (buzzing sound) is generated due to mismatch. 
From the causes of the failure caused by thinking: things have their own laws, equipment and equipment have their strict standards, large to the host, as small as a screw, only in accordance with the norms and standards of configuration and use, maintenance can ensure its normal operation, Not to be sloppy. Another point is to establish a strict procurement system to plug loopholes in the flow of inferior products into medical units.
Symptom 2 does not pass calibration. 
Analysis and Maintenance Based on experience, the reasons for calibration failure are mostly reagent problems. Because the quality of the reagents is directly related to the accuracy of the test results. The use of the reagent package for quality control checks, to determine the reagent is no problem. Excluding the reagent problem, the rest is equipment problems and the operation of medical technicians. The first thing to be solved is whether there is any problem in the operation of the user. Check the setup program. Set up a replacement kit with a 400 ml reagent pack. The setup procedure should be: Replace reagent? - Yes, reagent zero? - Yes, 400ml? - Yes, 800ml? - No. Followed by re-calibration, calibration results: calibration passed. In reverse analysis of the original calibration can not be passed because of the wrong settings. The error procedure is: Replace the reagent pack? - Yes, zero reagent pack? - Yes, 400ml? - No, 800ml? - Yes. As a result, the calibration must be correct, because 400 ml reagent packs are replaced, and 400 ml and 800 ml reagent kits match the two standards A and B. The interface positions of the two standards are different. When the program is set incorrectly, it will inevitably cause the machine to check the B concentration with the A standard solution during the operation, and check the A concentration with the B standard solution. As a result, the above reagents cannot be calibrated.
Symptoms 3K/clmv decreases. 
The analysis and overhaul of the fault phenomenon is manifested as a procedural calibration that can enter the blood analysis state, but it cannot measure the K/CI result. The causes of this failure are roughly the following: 1 electrode aging; 2 pump tube relaxation, this situation is the main factor causing and affecting the mv of CI; 3K, CI electrode electrode protein deposits remain.
When overhauling, different methods should be taken according to different reasons. 
(1) In accordance with the conventional practice, generally proceed with the above-mentioned third kind of failure phenomena. The protein precipitates in the K and CI electrodes can be cleaned with an electrode clearer, and then rinsed with a rinse solution. It is also possible to use serum or diluted serum instead of rinsing fluid for cleaning because serum has the property of activating K, CI electrodes. If there is no protein residue in the electrode, calibration is usually performed after starting to measure the sample. The mv value of the K and CI electrodes will increase to normal. 
(2) Check the pump tube. If the mV value of the electrode CI is low and the pump tube is slack, it is determined to replace the pump tube. After the replacement, the normal mv value will return to normal. 
(3) The above two methods of maintenance still cannot restore the mv values ​​of K and CI to normal values, which means that the K and CI electrodes are seriously aged. Only the replacement of the electrodes can ensure the normal operation of the detection.
Symptom 4 Clearance, calibration, and air bubbles occur during sample injection. The instrument alarms "**** Air." 
Analysis and Maintenance According to the analysis of fault phenomena, it is generally considered whether the specimens are added as required and whether the reagent packs are placed according to regulations. However, these factors should be excluded because these are mandatory procedures, and the fault performance is clear, calibration, and sample injection. Bubbles cause device alarms. Therefore, the next thing to consider is whether the solution piping is faulty. From the fact that the machine alarms air with the fault, it should be judged that the solution pipeline is not smooth, and the greatest possible cause of the unobstructed in the pipeline is the solution valve. After confirming the fault location, remove the solution valve and inhale the sodium hypochlorite disinfectant with a dilution of about 3% with a larger syringe (pull the needle). Inject disinfectant into the holes below the solution valve to clean and dredge. During the operation, it is necessary to use moderate force, several times in succession, and then purge the valve to remove the washing liquid. Re-install the test. The general failure will be quickly eliminated, and the above failure phenomenon will not occur again. 
The fault is a common fault, and the reason is: one kind of situation is due to the short serum separation time of the test sample, the presence of coagulation micro-blocks, and the blockage in the solution valve. In the other case, the residual serum is not cleaned in time after the test blood sample is finished. The long-term retention in the device causes serum protein to deposit in the solution valve and clog its valve passage, causing an equipment failure alarm. The thinking triggered by this failure is that the daily maintenance of the equipment is an important guarantee for maintaining good equipment rate and high quality operation. The management and maintenance personnel do a good job in management and maintenance, and formulate the rules for use and maintenance according to the different adaptability characteristics of various equipments, guide the medical technicians to use and maintain the equipment in accordance with the regulations, and the equipment is also excellent in rate and quality. The important guarantee for the operation.
Medical Products Production Line refers to the whole process of medical consumables making. It usually includes many different steps, and kinds of machines are working together to make one finished product for sale or usage.
Take the Syringe Production Line as an example. It consists of injection and molding, printing, assembly, packing and sterilization, five steps in total. So Syringe Making Machine includes Injection Molding Machine, Syringe Printing Machine , Syringe Assembly Machine , Automatic Blister Packing Machine or Pillow Packing Machine and ETO Sterilization Machine.
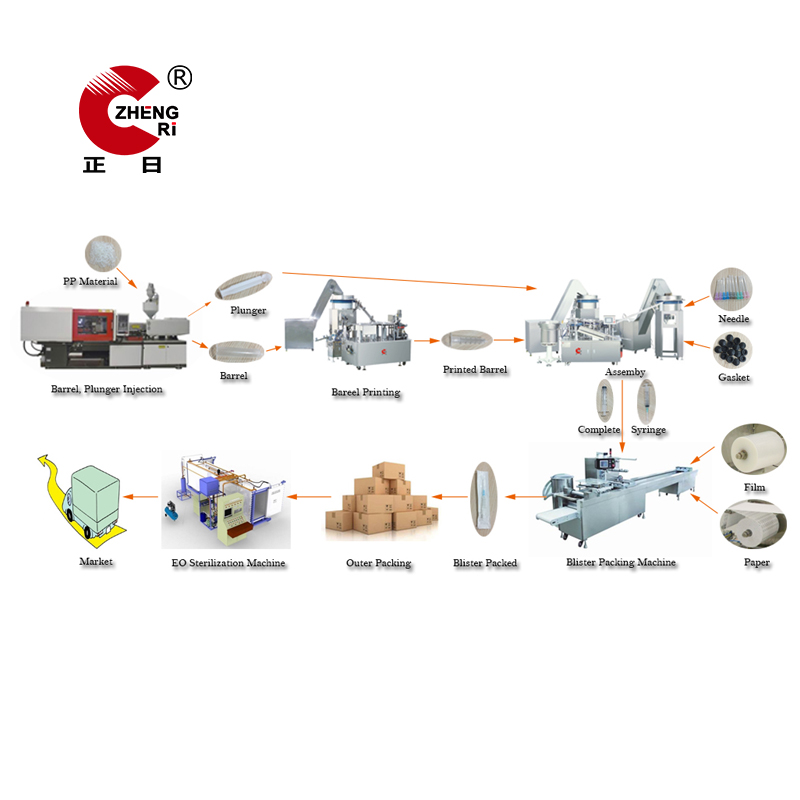
We offer the turnkey solution for various medical products production line, such as disposable plastic syringe production line as above, I.V set production line, and hypodermic Needle Production Line etc. If you`re interested, welcome to contact us at any time.
Medical Products Production Line
Medical Products Production Line,Production Line For Medical ,Medical Automatic Production Line,Medical Liquid Filling Production Line
Yuhuan Zhengri Technology Co., Ltd. , http://www.syringemachine.com